The strategic importance of structured reporting implementation levels
The six implementation levels of structured reporting, as defined in comprehensive research from the Royal College of Pathologists and validated through extensive radiological studies, form a progressive framework that defines the sophistication, standardization, and interoperability of medical documentation systems.
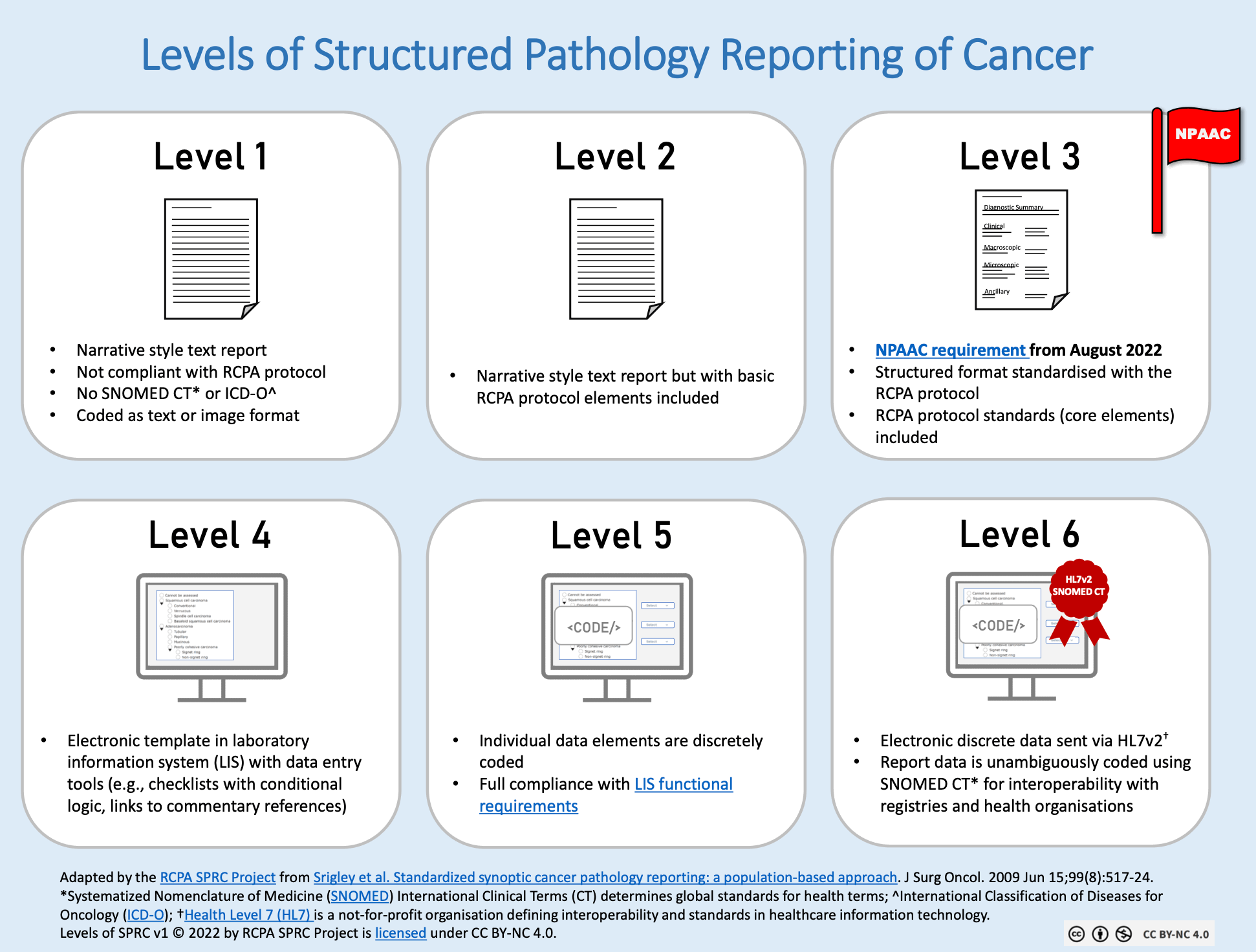
This classification, originally established through collaborative efforts between international medical organizations and refined through practical implementation studies, serves as more than an academic exercise. It provides healthcare organizations with a practical roadmap for digital transformation. Research demonstrates that institutions operating at higher implementation levels achieve superior clinical outcomes, enhanced operational efficiency, and stronger competitive positioning in an increasingly data-driven healthcare landscape.
Level 1: The foundation of narrative reporting
Characteristics: Reports in narrative language
Level 1 represents the traditional approach to medical reporting, where healthcare providers document their findings in free text. This method, while intuitive for physicians, brings significant challenges in terms of consistency and data extraction.
At this stage, specialists write their reports as they have for years: in natural language, using their own terminology and structure. A radiologist might write: "A small lesion is observed in the right upper lobe, possibly inflammatory in nature, follow-up recommended." This approach is flexible and allows physicians to express nuances and complex observations, but simultaneously creates problems for data analysis and interdisciplinary communication.
The primary limitations of level 1 include inconsistency between different reporters, difficulty extracting structured data for research or quality improvement, and potential misunderstandings due to variations in terminology and writing style.
56% of healthcare professionals still rely primarily on typing plain text directly into EHR systems
Our State of Structured Data research reveals that 56% of healthcare professionals still rely primarily on typing plain text directly into EHR systems, highlighting how prevalent this Level 1 approach remains across Belgian healthcare organizations. Despite widespread acknowledgment of structured data's value, many organizations continue to operate at this foundational level.
Level 2: Initial standardization with locally standardized content
Defining characteristics: Reports following institution-specific templates and guidelines
Level 2 marks the crucial first step toward systematic standardization, where healthcare institutions develop and implement internal reporting guidelines and templates. This evolution significantly improves consistency within organizational boundaries while establishing the foundation for more advanced structured reporting capabilities.
Organizations at this level typically develop department-specific reporting standards that reflect local clinical practices and preferences. A pathology department might establish that all colorectal biopsy reports must include standardized sections: specimen identification, macroscopic description, microscopic findings, immunohistochemistry results (when applicable), molecular studies (if performed), final diagnosis, and clinical recommendations. This systematization ensures comprehensive coverage of essential diagnostic elements while maintaining consistency across all departmental reports.
Benefits and limitations of local standardization
Advantages: Level 2 implementation delivers immediate improvements in report consistency, reduces omission of critical information, and facilitates more efficient interpretation by clinical colleagues within the same institution. Staff members develop familiarity with standardized formats, leading to faster report completion and improved clinical workflow efficiency.
Constraints: However, local standardization creates "institutional silos" where reports are highly consistent internally but may be structured entirely differently from comparable institutions. A breast cancer pathology report perfectly standardized according to Hospital A's criteria might organize information in a completely different sequence and format compared to an equivalent report from Hospital B, limiting interoperability and collaborative research opportunities.
Level 3: Structural advancement through criteria-driven organization
Defining characteristics: Reports structured according to standardized criteria and systematic frameworks
Level 3 represents a sophisticated evolution toward formal report architecture, where medical documentation follows predetermined structural criteria designed to optimize both human comprehension and computational analysis. This systematic approach ensures consistent information organization while facilitating advanced data mining and quality assessment capabilities.
At this implementation level, reports are methodically organized into discrete sections, each serving specific clinical and analytical purposes. A comprehensive cardiology report might be structured as follows: patient demographics and clinical context, examination indication and clinical question, technical parameters and imaging protocols, systematic anatomical evaluation (left ventricle, right ventricle, valvular structures, pericardium), quantitative measurements and calculations, comparative analysis with previous studies, diagnostic impression, and clinical recommendations with risk stratification.
Strategic advantages of criteria-driven structuring
Enhanced completeness: Systematic frameworks significantly reduce the likelihood of omitting critical diagnostic elements, as the structured format serves as a comprehensive checklist for reporting physicians.
Improved navigability: Standardized organization enables healthcare providers to rapidly locate specific information categories, enhancing clinical decision-making efficiency and reducing interpretation time.
Data analysis readiness: Consistently structured reports create opportunities for systematic quality assessment, outcome tracking, and population-level analysis that would be challenging or impossible with less organized documentation approaches.
Foundation for advancement: Level 3 implementation establishes the organizational and technological infrastructure necessary for progression to more sophisticated reporting levels, including electronic systems and international standardization.
Level 4: Digital transformation via electronic systems with intelligent validation
Defining characteristics: Electronic report creation through structured interfaces with comprehensive completeness verification
Level 4 represents a transformative technological advancement through the implementation of sophisticated electronic reporting platforms that guide users through systematic documentation processes while ensuring comprehensive coverage of all essential clinical elements. These intelligent systems combine user-friendly interfaces with robust quality assurance mechanisms to deliver superior reporting consistency and completeness.
Modern Level 4 platforms replace traditional free-text entry with carefully designed electronic interfaces featuring dropdown menus, standardized terminology selections, numerical input fields with validation ranges, and structured checklists. Advanced systems provide real-time feedback on report completeness, alert users to potential inconsistencies, and prevent finalization until all mandatory elements are properly addressed. For instance, a pathology report for invasive breast carcinoma might require completion of all TNM staging components, hormone receptor status, HER2 expression levels, Ki-67 proliferation index, and prognostic scoring before the system permits report validation and release.
Benefits of electronic structured reporting (forms)
Guaranteed completeness: Electronic systems eliminate the possibility of incomplete reports through systematic validation, ensuring that all critical diagnostic and prognostic elements are consistently documented across all cases.
Quality standardization: Digital platforms reduce inter-observer variability by providing standardized terminology options and guided decision trees, leading to more consistent diagnostic interpretations and improved clinical communication.
Integrated analytics: Electronic systems automatically capture structured data elements, enabling real-time quality metrics calculation, performance dashboards, and continuous improvement initiatives without additional manual data collection efforts.
Workflow optimization: Modern platforms integrate seamlessly with existing healthcare information systems, reducing documentation time while simultaneously improving report quality and clinical utility.
Level 5: International harmonization achieving global standardization excellence
Defining characteristics: Reports conforming to internationally recognized guidelines and protocols
Level 5 represents the achievement of true global interoperability through the adoption of internationally established reporting standards and guidelines. Organizations at this sophisticated level align their documentation practices with recognized international authorities, enabling seamless collaboration, research participation, and quality benchmarking across national and continental boundaries.
At this advanced implementation level, healthcare institutions adopt comprehensive reporting standards developed by prestigious international organizations such as the International Collaboration on Cancer Reporting (ICCR-Cancer), the College of American Pathologists (CAP), and other globally recognized medical societies. A breast carcinoma pathology report, for example, would strictly adhere to international consensus guidelines covering tumor size, histological grade, lymph node status, hormone receptor expression, HER2 status, lymphovascular invasion assessment, and margin evaluation. Ensuring that the report meets the highest global standards for clinical care and research applications.
Strategic advantages of international harmonization
Global research integration: Level 5 reports enable seamless participation in international multicenter studies, clinical trials, and collaborative research initiatives, as data elements are consistently defined and structured according to global consensus standards.
International care continuity: Patients receiving care across different countries or healthcare systems benefit from universally interpretable reports, facilitating optimal clinical decision-making regardless of geographic location.
Quality benchmarking: Organizations can meaningfully compare their clinical outcomes, diagnostic accuracy, and performance metrics against international cohorts, identifying opportunities for continuous improvement and best practice adoption.
Regulatory compliance: Many international accreditation bodies and research funding organizations increasingly require adherence to global reporting standards, making Level 5 implementation essential for institutional competitiveness and regulatory compliance.
Level 6: Ultimate interoperability through international codes integration and beyond
Defining characteristics: Reports integrating internationally recognized medical coding systems with advanced computational capabilities
Level 6 represents the pinnacle of structured reporting evolution, where medical documentation transcends traditional boundaries by seamlessly integrating sophisticated international coding systems with advanced computational capabilities. Tiro.health's revolutionary platform redefines what's possible at this level, delivering a comprehensive ecosystem that combines cutting-edge terminology management with intelligent workflow optimization.
The computational power of advanced medical coding
At Level 6, critical medical concepts are simultaneously expressed in natural language and precisely linked to internationally recognized coding systems, creating a rich semantic foundation for advanced healthcare analytics and artificial intelligence applications.
SNOMED CT (Systematized Nomenclature of Medicine - Clinical Terms) serves as the comprehensive foundation for clinical terminology standardization. When a pathologist diagnoses "invasive ductal carcinoma with moderate nuclear grade," Tiro.health's advanced system automatically links this to SNOMED CT code 395557000 while simultaneously capturing related semantic relationships, anatomical hierarchies, and clinical associations. This sophisticated approach eliminates diagnostic ambiguity and ensures consistent interpretation across all computational systems worldwide.
LOINC (Logical Observation Identifiers Names and Codes) provides precise standardization for laboratory observations and clinical measurements. A comprehensive metabolic panel isn't simply recorded as numerical values but systematically linked to specific LOINC codes that define the exact substance measured, specimen type, analytical method, and measurement scale. For instance, serum glucose measured via enzymatic methodology becomes LOINC code 15074-8, enabling precise data integration and comparative analysis across different laboratory systems and institutions.
ICD-O (International Classification of Diseases for Oncology) delivers specialized oncological coding that precisely defines tumor behavior, histological characteristics, and anatomical locations. This sophisticated coding enables advanced cancer surveillance, epidemiological research, and outcomes analysis that would be impossible with less structured approaches.
Tiro.health's Level 6+ capabilities
Tiro.health's advanced platform transcends traditional Level 6 limitations by delivering unprecedented capabilities that set new standards for structured reporting excellence:
Intelligent semantic interoperability: Unlike basic Level 6 solutions, Tiro.health's reports achieve seamless integration across diverse healthcare ecosystems through advanced FHIR standard compliance, automatic ontology mapping, and intelligent data validation systems. Our platform ensures perfect data integrity during exchanges between research databases, quality registries, and clinical information systems while providing comprehensive audit trails and version control.
Practical benefits of level 6 implementation
Semantic interoperability: Reports can be automatically exchanged between different computer systems without loss of meaning. Unlike basic level 6 solutions, Tiro.health's reports are designed for seamless integration across multiple platforms, automatically conforming to FHIR standards and enabling direct import into research databases, quality registries, and clinical information systems with enhanced data integrity checks.
Research readiness: Data from Tiro.health's platform is not only suitable for research but optimized for it. Our advanced data structuring goes beyond basic level 6 requirements, providing researchers with clean, validated datasets that significantly accelerate study timelines compared to traditional solutions.
Precision medicine enablement
The structured, coded data generated by advanced reporting systems plays a crucial role in enabling precision medicine initiatives. By systematically linking clinical observations to standardized terminologies, healthcare organizations create the rich datasets necessary for developing personalized treatment approaches based on individual patient characteristics, genetic profiles, and treatment response patterns.
Tiro.health's platform facilitates these precision medicine applications through sophisticated data integration capabilities that combine clinical reporting data with genomic information, treatment response metrics, and outcome measurements to create comprehensive patient profiles that support increasingly individualized care approaches.
Value-based healthcare enablement
The structured, coded data generated by advanced reporting systems serves as the essential foundation for implementing value-based healthcare (VBHC) initiatives. As healthcare systems worldwide transition from fee-for-service to fee-for-value models, the ability to systematically measure, analyze, and improve patient outcomes becomes critical for organizational success and sustainability.
Value-based healthcare, as defined by Harvard professors Michael Porter and Elizabeth Teisberg, fundamentally changes how healthcare providers are compensated—shifting from payment based on the volume of services delivered to payment based on patient health outcomes achieved. This transformation requires rigorous, disciplined measurement capabilities that can only be achieved through sophisticated structured reporting systems.
The International Consortium for Health Outcomes Measurement (ICHOM) has established global Standard Sets of outcome measures specifically designed to unlock VBHC potential. These standardized outcome measurements require precisely the type of structured, coded data that Level 6+ reporting systems generate. Healthcare organizations using advanced structured reporting platforms can seamlessly capture the condition-specific, multidimensional outcome data necessary for VBHC compliance and optimization.
Tiro.health's platform directly supports VBHC implementation through comprehensive outcome measurement capabilities that integrate clinical reporting with real-world evidence collection. Our system enables healthcare organizations to systematically capture the structured data required for ICHOM Standard Sets while simultaneously reducing administrative burden on clinical staff.
The platform's advanced analytics capabilities enable continuous quality improvement through real-time outcome tracking, benchmarking against international standards, and identification of improvement opportunities—all essential components of successful VBHC implementation that directly translate to better patient care and enhanced organizational financial performance.
Global collaboration and research acceleration
Level 6+ implementation creates unprecedented opportunities for international collaboration in clinical research and care delivery. When healthcare institutions utilize harmonized, coded reporting systems, they can participate in large-scale, multinational studies that would be impossible with less structured documentation approaches.
Tiro.health's advanced interoperability capabilities enable seamless participation in global research networks, international quality benchmarking initiatives, and collaborative care programs that transcend traditional geographic and institutional boundaries.
Conclusion: transforming healthcare through intelligent structured reporting
The evolution from basic narrative documentation to sophisticated, internationally coded reporting systems represents one of the most significant advances in modern healthcare information management. This systematic progression through implementation levels provides healthcare organizations with a clear roadmap for achieving excellence in clinical documentation, operational efficiency, and patient outcomes.
Tiro.health makes this transformation accessible through three core capabilities that work seamlessly together:
- Create medical forms:
Build standardized templates with our no-code designer, complete with AI-powered form creation and terminology integration - Fill them in efficiently:
Capture structured data through flexible input methods including our new voice synoptic reporting - Exchange your data easily:
Share research-ready, FHIR-compliant data across systems and participate in multicentric studies
While achieving Level 6 compliance represents a significant accomplishment, the choice of implementation platform determines whether organizations merely meet basic standards or achieve transformative capabilities that drive competitive advantage and clinical excellence.
Sources and References:
This article draws upon established research frameworks from leading international medical organizations:
- Structured Radiology Reporting: Addressing the Communication Quality Gap. Available from: https://www.researchgate.net/figure/Six-levels-of-structured-reporting-34_fig4_332595215
- Royal College of Pathologists of Australasia. Levels of SPRC infographic 2022. Available from: https://www.rcpa.edu.au/getattachment/a85def5c-a130-4cad-a710-f15363dcb2fd/Levels-of-SPRC-infographic-2022.aspx
- Tiro.health State of Structured Data Research: https://stateofstructureddata.com



