Introduction
The digital transformation of healthcare is fundamentally changing how medical information is captured, processed, and shared across healthcare systems. Where clinical documentation traditionally consisted of narrative reports filled with free text descriptions, we now see a decisive shift toward structured reporting. A methodology that dramatically improves the accuracy, completeness, and interoperability of medical data.
As we explored in our comprehensive guide to the power of structured reporting in healthcare, this evolution represents more than just a technological upgrade. Structured reporting, also known as synoptic reporting, uses standardized data elements and coded response options to create consistent, complete, and computable reports that deliver measurable benefits for healthcare organizations.
SNOMED Clinical Terms (SNOMED CT), the world's most comprehensive clinical terminology, plays a pivotal role in this transformation by providing a common language for medical concepts globally. Combined with modern interoperability standards, this creates an ecosystem where healthcare data can seamlessly flow between systems while maintaining its clinical meaning and diagnostic value.
Understanding this transformation is crucial for healthcare professionals navigating Belgium's new regulatory landscape. As outlined in our analysis of Belgium's new vision for healthcare registries, structured data capture at source is becoming not just advantageous but mandatory for compliance with European Health Data Space regulations taking effect in 2027.
Understanding structured reporting fundamentals
What is structured reporting?
Structured reporting refers to the practice of recording clinical findings using predefined data elements and standardized response options. Instead of writing a paragraph to describe a tumor, a pathologist selects specific options such as "Tumor size: 2 cm," "Histologic type: adenocarcinoma," and "Margins: clear" from a template. Each response links to a specific code (for example, a SNOMED CT concept) enabling computers to parse and analyze the information systematically.
This systematic approach aligns perfectly with the healthcare standards landscape we detailed in our health technology standards 101 guide. Rather than operating in isolation, structured reporting represents part of a broader ecosystem of interoperable healthcare technologies designed to work together seamlessly.
The evolution of medical reporting
The movement toward structured documentation began in the 1990s with cancer registries and tumor boards demanding more consistent data. This evolution progressed through distinct maturity levels, which we've comprehensively documented in our analysis of the power of structured reporting:
Level 1: Narrative reports → Unstructured text with no standardization, where information is captured entirely in free form.
Level 2: Standardized headings → Narrative text grouped under standard headings such as Clinical Information, Gross Description, and Diagnosis.
Level 3: Synoptic free text → Templates with discrete headings but free text answers, improving completeness while limiting computer comprehension.
Level 4: Synoptic structured reporting → Templates with both standardized headings and structured options, where each data element and response links to SNOMED CT codes.
Level 5: Integrated decision support → Structured reporting integrated with clinical decision support and quality metrics.
Level 6: Learning health system → Structured data feeds machine learning models and research databases that subsequently inform clinical decision making.
The momentum behind synoptic reporting (levels 3 and 4) grew significantly after professional societies developed checklist based cancer protocols. A randomized study of surgical pathology reports demonstrated that template based synoptic reports achieved 98% completeness versus 77% for narrative reports, establishing the clinical superiority of structured approaches.
SNOMED CT: The terminological foundation
Overview and hierarchical structure
SNOMED CT represents a systematically organized collection of medical terms used to encode clinical information in health records. As detailed in our health technology standards guide, SNOMED CT serves as one of the most crucial terminological standards in modern healthcare, providing the vocabulary backbone that enables true semantic interoperability.
The hierarchical structure organizes concepts in multiple parent child relationships. For example, Adenocarcinoma (morphologic abnormality) is a child of Carcinoma, which in turn is a child of Malignant neoplastic disease. This systematic organization enables both precise coding and sophisticated querying capabilities that support clinical decision making and research.
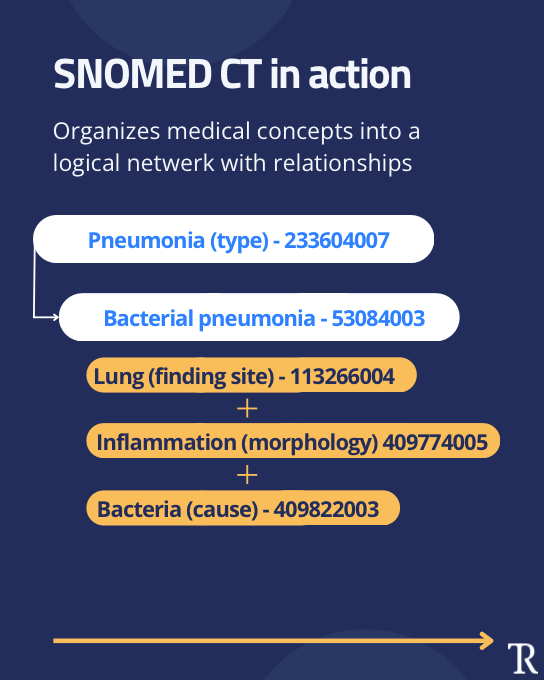
Each concept's comprehensive metadata includes:
- Fully specified name (FSN): A precise definition such as "Adenocarcinoma of colon"
- Preferred term: The everyday clinical term like "Colon adenocarcinoma"
- Synonyms: Alternative names and abbreviations
- Relationships: Links to parent concepts and defining attributes
This rich metadata enables SNOMED CT concepts to be mapped to other coding systems including ICD-10, LOINC, and MedDRA. As we explored in our ultimate guide to health data standards, these mappings ensure that SNOMED coded data can be reported to regulatory agencies and billing systems without semantic loss.
Integration with FHIR and modern standards
Structured reporting depends on more than terminology alone. To transmit structured data effectively, we need comprehensive standards for content, transport, and APIs. This is where FHIR (Fast Healthcare Interoperability Resources) becomes essential, as we detailed in our FHIR starters guide.
SNOMED CT integrates seamlessly with:
HL7 Clinical Document Architecture (CDA): An XML based standard for clinical document structure that defines templates for various document sections. Combined with SNOMED CT for coding, CDA enables machine readable, interoperable documents.
Fast Healthcare Interoperability Resources (FHIR): A modern API specification that defines resources for clinical data. Synoptic reports can be represented using FHIR resources such as Observation and DiagnosticReport, enabling data exchange across systems via RESTful APIs.
LOINC: A coding system for laboratory and clinical observations that complements SNOMED CT. While SNOMED CT encodes clinical meaning, LOINC identifies the observation itself, creating a comprehensive coding framework.
The relationship between these standards becomes particularly important when implementing multicentric studies, as we demonstrated in our multicentric quality benchmarks project. FHIR profiles ensure that data captured in resources is consistent and complete, essential for reliable cross institutional benchmarking.
Clinical benefits of structured reporting
Enhanced completeness and quality assurance
One of the most compelling arguments for structured reporting lies in its dramatic impact on data completeness. Studies across pathology, surgery, and radiology consistently demonstrate that synoptic reports capture significantly more mandatory data elements than narrative reports. As we documented in our comprehensive analysis of structured reporting benefits, the evidence is overwhelming: template based synoptic reports achieved 98% completeness versus 77% for narrative reports.
In oncology, precisely capturing tumor size, margins, and lymph node status is essential for accurate staging and treatment planning. Missing data can lead to under staging or incorrect treatment decisions, directly impacting patient outcomes. This systematic approach to data capture has proven particularly valuable in our multicentric studies, where consistency across institutions is paramount for meaningful analysis.
Improved interoperability and data exchange
Structured reports encoded with SNOMED CT facilitate unprecedented interoperability between healthcare systems. Because each data element links to standardized codes, different electronic health record (EHR) systems can exchange and interpret information consistently across institutional and geographic boundaries.
This capability proves particularly valuable for the European context, where cross border collaboration and data sharing are increasingly important. As outlined in Belgium's healthcare registry vision, the move toward federated data systems requires robust interoperability standards that SNOMED CT and FHIR provide.
Support for research and quality improvement
Structured data proves exponentially easier to aggregate and analyze for research purposes. This becomes particularly evident in our multicentric benchmarking initiatives, where standardized data capture enables meaningful comparison of outcomes across multiple institutions.
Cancer registries rely on standardized reporting to measure outcomes and identify trends across populations. SNOMED CT's hierarchical structure allows researchers to query broad categories (such as all breast cancers) or highly specific subtypes (such as triple negative ductal carcinoma) with precision, supporting both population health initiatives and targeted clinical research.
Implementation strategies and best practices
Designing effective synoptic templates
Implementing structured reporting requires thoughtful template design based on established clinical guidelines. Templates should incorporate national or international standards and include all mandatory data elements. Each data element must link to a SNOMED CT concept and, where appropriate, a LOINC code.
This systematic approach to template design aligns with the broader healthcare technology landscape we explored in our health technology standards overview. Understanding how different standards work together from terminology (SNOMED CT) to information models (FHIR) to data exchange protocols is crucial for successful implementation.
FHIR based implementation approaches
When implementing FHIR based synoptic reporting, each data element can be represented as a FHIR Observation resource with codes drawn from LOINC and valueCodeableConcept drawn from SNOMED CT. The overall report is encapsulated in a DiagnosticReport resource, enabling comprehensive data exchange.
Our FHIR starters guide provides detailed technical guidance on implementing these standards effectively. The key is understanding that FHIR serves as the transport and structure layer, while SNOMED CT provides the semantic meaning that ensures clinical information is preserved across system boundaries.
Overcoming implementation challenges
Common obstacles and solutions
Despite compelling benefits, structured reporting adoption faces several persistent challenges. Legacy system integration tops the list, as many healthcare institutions operate EHRs not designed for structured templates. Financial constraints and clinical resistance also create adoption barriers.
However, as demonstrated in our multicentric quality benchmarks project, these challenges are surmountable with proper planning and stakeholder engagement. The key is demonstrating tangible value while providing adequate training and support during the transition period.
Policy drivers and enablers
Several factors facilitate successful structured reporting adoption. Belgium's regulatory landscape, as detailed in our analysis of the new healthcare registry vision, increasingly mandates structured data capture at source. This regulatory pressure, combined with financial incentives and accreditation requirements, creates a compelling business case for adoption.
Advanced implementation: The comparison with OMOP CDM
While FHIR and SNOMED CT provide excellent solutions for real time data exchange and clinical documentation, organizations also need to consider long term data storage and research applications. As we explored in our comparison of FHIR and OMOP CDM for healthcare interoperability, different standards serve different purposes in the healthcare data ecosystem.
OMOP CDM (Observational Medical Outcomes Partnership Common Data Model) excels at standardizing data for large scale observational studies, while FHIR optimizes real time data exchange. Understanding when to use each standard and how they complement rather than compete with each other is crucial for comprehensive implementation strategies.
Our OMOP starters guide provides additional context for organizations considering this complementary approach to data standardization.
Future innovations and developments
AI and ambient intelligence integration
The future of structured reporting increasingly involves artificial intelligence and ambient technologies. Our exploration of ambient AI scribe technology reveals how voice enabled documentation can work alongside structured templates to optimize clinical workflows.
Rather than replacing structured reporting, ambient AI enhances it by reducing documentation burden while maintaining the precision and interoperability that structured data provides. This hybrid approach represents the future of clinical documentation, combining the efficiency of voice input with the analytical power of structured, coded data.
Emerging standards and regulations
As healthcare becomes increasingly digitized and globalized, staying current with emerging standards becomes crucial. Our ultimate guide to health data concepts provides ongoing insights into new developments in healthcare terminology, interoperability standards, and regulatory requirements.
Tiro.health's comprehensive solution
At Tiro.health, we've developed a comprehensive platform that addresses every aspect of structured reporting implementation. Our solution seamlessly integrates SNOMED CT terminology with FHIR based data exchange, creating a unified ecosystem for clinical documentation that meets both current needs and future regulatory requirements.
Key capabilities include:
- Source based structured data capture: Intuitive templates that integrate naturally into clinical workflows
- Comprehensive terminology support: Built in SNOMED CT, LOINC, and ICD-10 integration with automatic mapping
- FHIR native architecture: Full compliance with modern interoperability standards
- Multicentric research support: Seamless data sharing for quality benchmarking and research initiatives
- Regulatory compliance: Built in support for European Health Data Space requirements
Our multicentric quality benchmarks project demonstrates these capabilities in action, showing how structured reporting can drive both clinical excellence and research advancement simultaneously.
Frequently asked questions
What distinguishes structured reporting from traditional narrative reporting?
Structured reporting utilizes predefined data elements and coded response options to capture clinical information systematically, while narrative reporting relies on free text descriptions. Structured reports ensure completeness and enable computer processing for improved research and quality measurement, whereas narrative reports may omit essential data and prove difficult to aggregate across systems.
How does SNOMED CT support structured reporting implementation?
SNOMED CT provides a standardized vocabulary with unique identifiers, comprehensive synonyms, and hierarchical relationships. When each data element and response in synoptic templates links to SNOMED CT codes, the resulting reports become unambiguous and fully interoperable across different healthcare systems and geographic regions.
Why are synoptic reports particularly important for cancer care?
Cancer treatment depends heavily on accurate staging and comprehensive pathology information. Synoptic reports systematically capture all necessary elements including tumor size, surgical margins, and lymph node status, improving completeness compared with narrative reports. This consistency directly supports treatment planning, clinical trial enrollment, and cancer registry reporting.
What technical standards are required for structured reporting implementation?
Beyond SNOMED CT for terminology, standards such as HL7 CDA and FHIR define how structured data is represented and transmitted between systems. LOINC codes identify specific laboratory or clinical observations, while FHIR's RESTful APIs enable real time data exchange and integration with existing healthcare information systems.
How can healthcare organizations overcome resistance to structured reporting adoption?
Successful adoption strategies include early stakeholder engagement, demonstrating tangible efficiency gains, providing comprehensive training, and integrating templates seamlessly into existing clinical workflows. Pilot projects with feedback loops help refine implementations, while policy mandates and accreditation requirements provide external motivation for organizational change.
Conclusion: The path forward
Structured reporting with SNOMED CT represents more than a technological upgrade; it constitutes a fundamental transformation in how healthcare organizations capture, share, and utilize clinical information. The convergence of regulatory requirements, technological capabilities, and clinical benefits creates an unprecedented opportunity for healthcare organizations to modernize their documentation practices.
Success requires understanding how different standards work together in the broader healthcare technology ecosystem. From basic health technology standards to advanced FHIR implementation strategies, each component plays a crucial role in creating interoperable, analyzable, and clinically meaningful documentation systems.
The evidence from our multicentric benchmarking initiatives demonstrates that organizations implementing comprehensive structured reporting solutions see immediate benefits in data quality, clinical workflow efficiency, and research capabilities. More importantly, they position themselves for success in an increasingly regulated and interconnected healthcare environment.
For healthcare professionals and organizations ready to embrace this transformation, the path forward is clear: implement structured reporting now, leverage proven standards like SNOMED CT and FHIR, and partner with tiro.health who understand both the clinical and technical requirements of modern healthcare documentation.
The future of healthcare depends on our ability to capture, share, and learn from data. Structured reporting with SNOMED CT provides the foundation for that future, enabling the learning health systems that will drive continuous improvement in patient care and clinical outcomes.