Why structured reporting is becoming essential for pathology departments
A new paper published in Annales de pathologie makes a compelling case for standardized structured reporting in pathology. The authors, Jean-Pierre Bellocq and Dominique Fétique, argue that while traditional narrative reports have served pathology well, the field is at a turning point where structured data capture is becoming indispensable.
The current state of pathology
Pathology departments face unprecedented challenges. The paper identifies several converging pressures: implementation of digital imaging in daily practice, spectacular developments of AI-based diagnostic tools, continuous growth of medical information from diverse sources to interpret and share during complex care pathways, increased workload partly related to limited staffing, and accelerating medical knowledge that doubles faster than ever before.
In this environment, even high-quality narrative reports reveal their shortcomings. The authors note that less than 5% of cancer pathology reports in France currently reach what they define as level 3 structuring, a standardized PDF format. Most reports remain at level 1: essentially narrative text organized into paragraphs.
This aligns with what we have observed for years at Tiro.health through our multicentric quality benchmarking projects, where data quality forms the fundamental prerequisite for meaningful comparisons between healthcare institutions.
Six levels of report structuring
The paper provides a useful framework for understanding different approaches to structuring. This framework corresponds closely to the levels we describe in our comprehensive analysis of the power of structured reporting:
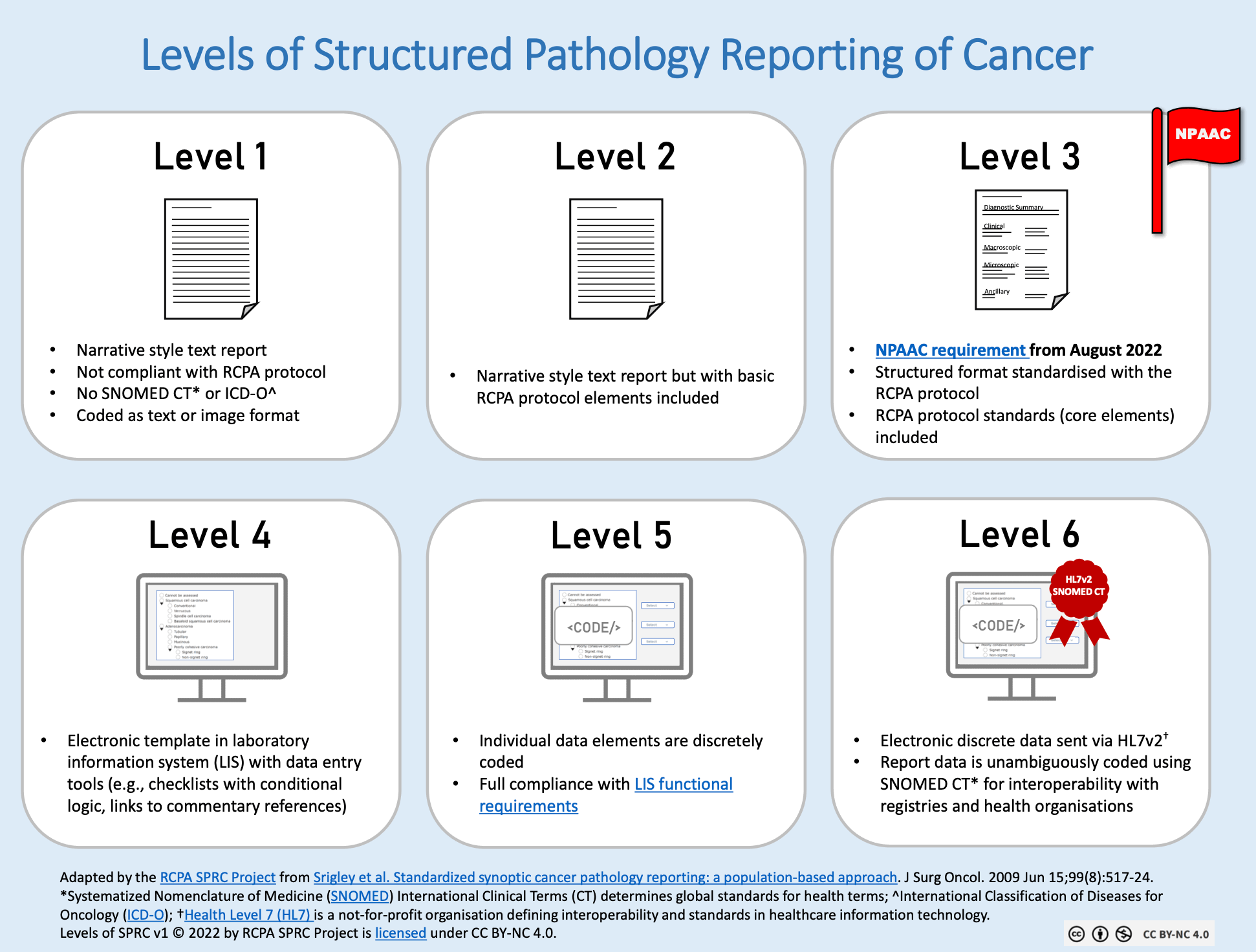
Level 1 corresponds to traditional narrative text organized into chapters or paragraphs. This is by far the most commonly used form but offers minimal possibilities for automated processing.
Level 3 represents fine, standardized structuring in non-queryable PDF format, as proposed by organizations like the ICCR (International Collaboration on Cancer Reporting).
Levels 5 and 6 represent true computer-readable data with queryable fields. At level 6, these fields are linked to international terminologies like SNOMED CT, the global standard for medical terminology encompassing more than 350,000 active concepts.
The Netherlands, through PALGA, has implemented level 5 structuring nationwide. France is now pursuing similar ambitions through the Impulsion initiative. Belgium has set its own ambitious timeline, as outlined in Belgium's new vision for healthcare registries.
Structured reporting outperforms AI extraction
One of the most striking findings concerns the comparison between prospective structured reporting and retrospective data extraction using natural language processing. Despite remarkable advances in generative AI, structured reports created at the source remain more reliable.
Hewer et al. reported an error rate of 8.7% when using an AI-based large language model to extract data from narrative (free-text) melanoma reports, compared to 0.05% error rate for manual extraction from structured reports. A 174-fold difference in error rate.
This finding has significant implications for healthcare organizations investing in AI solutions. While NLP can retrospectively structure existing narrative reports, the quality of that structured data will inherently be lower than data captured in structured form from the beginning.
Evidence for improved patient outcomes
Perhaps the most compelling argument for structured reporting comes from patient outcome data. The paper cites a Dutch study of 72,859 patients with colorectal carcinoma that found clinical use of structured reporting was optimized due to more complete content: 95.8% for structured reports versus 89.8% for narrative reports.
More importantly, the 5-year survival rate was significantly improved for patients who benefited from structured reporting: 64.9% versus 62.2%. Even after controlling for variables such as stage, grade, and therapy, structured reporting's advantages persist, with a hazard ratio of 0.94 indicating a significant enhancement in survival outcomes.
The mechanism likely relates to more complete and accurate information exchange throughout the care chain, enabling more appropriate treatment decisions. This echoes what our own multicentric benchmarking initiatives have demonstrated: when data is captured consistently across institutions using standards like FHIR and SNOMED CT, meaningful quality comparisons become possible and drive improvements in clinical practice.
Barriers to adoption and how to overcome them
The authors acknowledge that structured reporting faces real obstacles. Pathologists have described it as insufficiently flexible, not user-friendly, and a source of time loss in daily production. Situations of diagnostic uncertainty and the expression of clinical doubt can be particularly challenging to capture in structured formats.
The paper suggests that overcoming these barriers requires tools built on open technologies with adaptable forms that can handle both complex and routine cases. Regular updates to template repositories, integration with digital imaging, and AI-assisted pre-filling of forms based on lesion identification could all help ease adoption.
This is precisely the approach Tiro.health has taken. Our platform addresses these concerns through intuitive templates that integrate naturally into clinical workflows, comprehensive terminology support with built-in SNOMED CT, LOINC, and FHIR-native architecture for seamless data exchange.
The convergence of structured reporting, digital pathology, and AI
The convergence of structured reporting, digital pathology, and artificial intelligence opens significant possibilities. Structured pathology data correlated with multimodal sources of clinical, radiological, and biological information will benefit both clinical care and research.
The concept of population-based digital twins, while still nascent, relies fundamentally on the precision and granularity that structured data provides. These augmented data will contribute to creating patient profiles through specific parameters to simulate group behaviors, proving particularly interesting for clinical trials by reducing their duration and cost, or in personalized medicine to determine optimal treatment and predict response to treatment.
As healthcare becomes increasingly digitized and globalized, staying current with emerging standards becomes crucial. Our ultimate guide to health data concepts provides ongoing insights into developments in healthcare terminology, interoperability standards, and regulatory requirements including the European Health Data Space.
What this means for pathology departments
For pathology departments considering their data strategy, this paper reinforces what many have suspected: the investment in structured data capture at the source will pay dividends not just for research and quality improvement, but potentially for patient outcomes as well.
Healthcare organizations should evaluate their current terminology usage and identify departments with existing structured reporting experience. Selecting priority use cases and developing pilot programs allows teams to demonstrate value while refining workflows. Integration with existing electronic health record systems through standards like FHIR ensures that structured data flows seamlessly throughout the care chain.
As Hewer emphasizes in the paper: "For precision medicine not to remain an empty promise, precision must be the modus operandi of all oncological medicine practice, and synoptic reports are the most precise type of communication at our disposal."




